Finding the right contract medical device manufacturing partner for your medical device production is the key to getting the most successful results. The benefits to be had include increased efficiency, productivity and profitability. But, how do...
Medical device design & development
Categories
Is it better to update an existing device or create a new one?
13/06/2023
In the medical industry, innovation is key to meeting patient needs and improving health outcomes. As such, medical device manufacturers are constantly faced with the question of whether to develop a new medical product or update an existing...
How are medical devices approved in the UK?
23/05/2023
When launching a new medical device or updating the specification to an existing product, your company needs to ensure that it has the correct regulatory approvals before releasing the device for human use or general sale. In the UK, medical...
Why less is more when it comes to medical device manufacturing
11/04/2023
Medical device manufacturing is a complex and highly regulated industry that requires a great deal of attention to detail. One of the key principles that manufacturers should keep in mind is that less is more when it comes to designing,...
5 steps to protecting intellectual property in medical device development
13/07/2021
In a marketplace characterised by increased globalisation, fast-paced innovation, fierce competition and stringent regulation, the protection of product intellectual property is a key priority for medical device companies. Therefore, when...
6 ways a contract medical device manufacturer can assist with product development
15/06/2021
More and more medical device manufacturers are outsourcing the product development part of the manufacturing process to contract medical device manufacturers. A vital part of the manufacturing process, the standard of product development has a...
The 12 phases of medical device development
11/05/2021
A key area of medical device manufacturing is the development of new and improved medical products. Much of the development process can be outsourced to a contract medical device manufacturer but it is critical that you choose the right partner,...
The benefits of working with a specialist medical device manufacturer
10/08/2020
When you outsource your medical device manufacturing, it is crucial that you choose the right partner - one that you can trust and that will make your business stronger. It’s a critical choice. The decision that you make will have a direct impact...
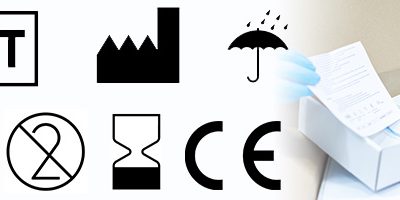
12 key signs and symbols on medical devices and packaging
09/06/2020
In medical device manufacturing, a medical device’s label must clearly display the necessary information to ensure not only the safety of the end user but also legal compliance. This includes a series of key signs and symbols, which should be...